Quality you can trust
To assure the highest quality and to comply with the new Indian Medical Device Rules 2017 (MDR) we implemented a Quality Management System that is evaluated, approved and certified according to ISO 13485.
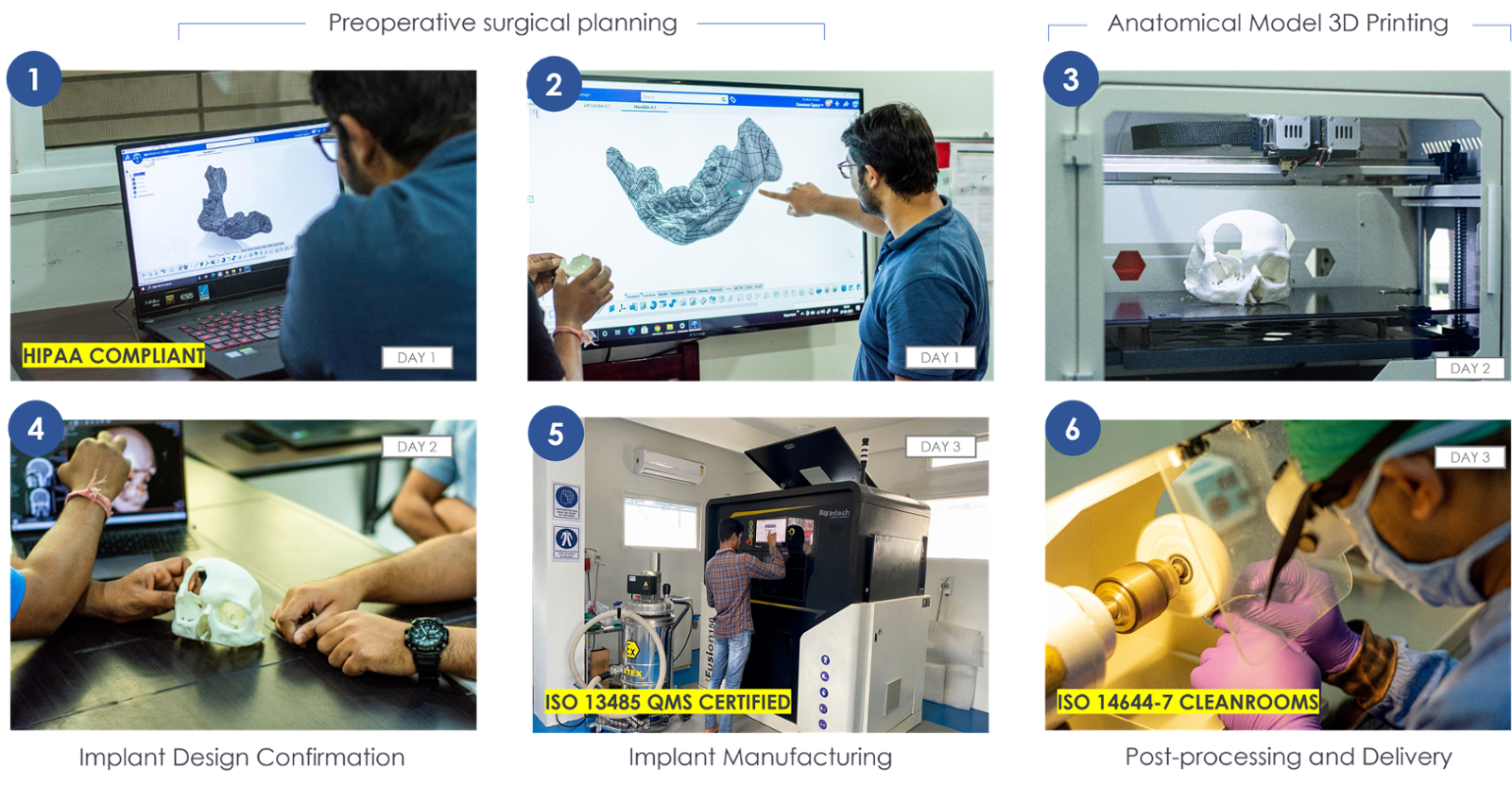
We developed a proprietary connected quality platform where every record of your case is organized, centralized, and end-to-end traceable. This helps track and manage quality events and associated follow up tasks digitally, drive traceability throughout quality, design, and risk processes and easily manages documentation for audit, anytime.
